Friday, July 6, 2018
True Confessions On Why I Prescribe Things Without 'Evidence'
We have a 'required reading' list for our fellowship, which includes a bunch of what I think are landmark or otherwise really important studies. One of them is this very well done RCT of continuous ketamine infusions for patients with cancer pain, which showed it to be ineffective (and toxic).
We also recently have seen another high-quality study published with negative results for ketamine. This was a Scottish, multi-center, randomized, placebo-controlled, intention-to-treat, and double-blinded study of oral ketamine for neuropathic pain in cancer patients. The study involved 214 patients, 75% of whom were through cancer treatments and had chemotherapy-induced peripheral neuropathy (CIPN), and the median opioid dose was 0 mg. They received an oral ketamine (or placebo), starting at 40 mg a day, with a titration protocol, and were followed for 16 days.
There were exactly zero measurable differences in outcomes between the groups (on pain, mood, or adverse effects). Zip.
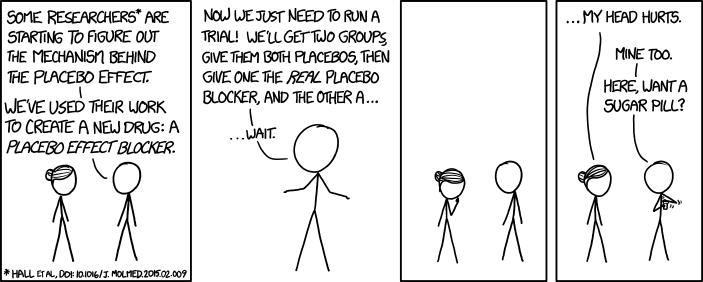
All this got me thinking about a conversation I had with a palliative fellow this year, who, upon reading the continuous infusion study, confronted me with the question - Why do you even still use ketamine, then? The answer to this has a lot to do with the nature of evidence and how that is different for symptom management than it is for other outcomes, as well as the challenging reality of the placebo effect in everything we do.
I should note that you can 'dismiss' these studies based on generalizability (and plenty of people do), i.e., "The infusion study was well-done, but it's a protocol that I don't use, therefore I can ignore it." This very detailed letter to the editor about the infusion study does just that, for instance. Or, that the oral ketamine study was really a study about CIPN, and virtually nothing has been shown to be effective for CIPN, except maybe duloxetine (barely), and so it's not generalizable beyond that, and can be summarily ignored.
All this is valid, to be sure -- it's always important to not extrapolate research findings inappropriately, but honestly the reason I still prescribe ketamine sometimes has little to do with this, and has everything to do with the fact that I have observed ketamine to work and believe it works despite the evidence. Which is a pretty uncomfortable thing to admit, what with my beliefs in science, data, and evidence-based medicine.
Perhaps.
The challenge here is that when it comes to symptom treatments us clinicians are constantly faced with immediate and specific data from our patients as to whether our treatments are working. This is a very different situation than a lot of other clinical scenarios for which we lean heavily on research statistics to guide us. Note that it's not a bad thing we're confronted with this data (!), it just makes it difficult to interpret research sometimes.
Let's start with research which involves outcomes which are not immediate. E.g., does statin X, when given to patients for secondary prevention of myocardial infarction, actually reduce the number of myocardial infarctions (MI) or prolong survival? We can only answer that question with research data because when I give statin X to an actual patient, I have zero way of knowing if it is 'working.' If they don't ever have another MI I'll feel good, but that may take years and decades to even find out. In fact, it's nearly incoherent to even talk about that outcome for my patient, because we think about those sorts of outcomes as population outcomes because that's how they are studied. E.g, we know that if we give statin X or placebo to a population of patients (who meet certain criteria) for Y number of years, that the statin X group will have some fewer number of MIs in it than the placebo group. That's what we know. And because some patients in the placebo group don't have MIs and some in the statin X group do have MIs, we actually cannot even conclude for our own patient whether statin X helped them, even if they never have another MI, because maybe they wouldn't have had an MI anyway. That is, it's a population-based treatment, with outcomes that only make sense on the population level, even though of course we and our patients very much hope that they individually are helped by the drug. Supposedly precision/personalized medicine is going to revolutionize all this, and maybe it will, but it hasn't yet.
Contrast this to symptom management. My patient is on chemotherapy and they are constantly nauseated. I prescribe a new antiemetic -- let's call it Vitamin O just for fun. Two days later I call them up, and they tell me: "Thanks doc, I feel a lot better, no more vomiting and I'm not having any side effects from the med." Or they tell me: "Doc, the Vitamin O just made me sleep all day and it didn't help the nausea one bit." I have immediate, actionable, patient-specific, and patient-centered data at my fingertips to help me judge if the treatment is effective/tolerable/worth it. It feels very different than prescribing statin X, in which all I have is the population data to go by.
So then why do symptom research at all if all we have to do is just ask our patients?
Obviously, it's not that simple, and research is critically important. For one, placebo-effects are hugely important for symptom research, in fact, they dominate symptom research. Blinded and controlled studies are critical in helping us understand if interventions are helpful above and beyond placebo effects (we should all be skeptical/agnostic about any symptom intervention which is not studied in a blinded and adequately controlled manner). Research also helps us get a general idea of the magnitude of clinical effects of certain interventions. Comparative research (of which there's very little, but it's really important) helps guide us towards which interventions are most likely to be the most helpful to our patients. E.g., which antiemetic is most likely to help the largest number of my patients going through a certain situation (so as to avoid painful delays as we try out ineffective therapies)? Research also obviously helps us understand side effects, toxicities -- hugely important.
But...if I thought all of the above were sufficient, I'd still never prescribe ketamine, or for that matter methylphenidate, because the placebo-controlled, blinded studies don't actually indicate they are effective over placebo (let's be honest palliative people, when we actually read the high-quality methylphenidate studies, there's very little there to suggest we should ever prescribe it).
That leaves me though with this belief, based on patient observation, that it still works, damn the data. What do I make of that? I want to be clear, I don't prescribe ketamine a lot, just the opposite, but there are times when you are desperate, you are faced with a patient in an intractable, painful situation, and you're running out of moves to make to improve the patient's life, and the reality is I sometimes will prescribe ketamine then, and my observation is that it's sometimes hugely helpful, enough so that I keep on using it.
And I honestly don't know what this represents - is it that complex phenomenon called the placebo-effect that decides to show up every now and then (although for these patients you wonder why the placebo-effect didn't show up on the 5 prior treatments you threw at them)? Is it that I'm 'just' making them euphoric and I'm not actually helping their pain (although honestly, I think it's impossible to draw a hard line between the two)? Or is it the fact that for presumably complex genetic neurobiological reasons, while ketamine is ineffective toxic for the majority of patients out there, it is also really effective/well-tolerated for a minority of our patients, and that's the sort of thing that it's tough to parse out in trials, because the small number of responders is overwhelmed by the strong majority of non-responders.
I like to tell myself it's the latter, although I need to admit that probably a lot of the time it is placebo-effects. None of us should be happy about prescribing drugs with real side effects, and we must recognize the possibility for patient harm 'just' for placebo effects. (Which, incidentally, is why I'm perfectly ok using lidocaine patches sometimes even when I just assume it's a placebo - because of the near zero chance of harm to the patient. True confessions.)
But, to emphasize my point, if it is the latter (some drugs like ketamine and methlyphenidate do actually really help a minority of patients but are toxic to most and so it's tough to appreciate the impact based on clinical trial research), that emphasizes the critical observation about why high-quality clinical research is important - it helps us know which interventions we should be doing routinely and early, and which should be at the bottom of the bag, to be used rarely, and with great consideration.
But, given that this is true confessions day, I still don't think methylphenidate is something to be rarely used. In fact, it's one of the few things I do in which I routinely have patients/families enthusiastically tell me thank you that made a huge difference. (If you're curious those things are 1) talking with them empathetically and clearly about what's going on and what to expect with their serious illness, 2) starting or adjusting opioids for out of control pain, 3) olanzapine for nausea, and 4) methylphenidate.) Like, all the time. Like, they come back to see me in a couple weeks with a big smile on their face, so glad I started the methlyphenidate. Happens a lot (not all the time, but enough of the time). A lot more than with gabapentin or duloxetine or many other things I also prescribe all the time which have 'good evidence' behind them. It happens enough that I've asked myself What data would convince me to stop prescribing it to my patients? And I don't have an answer for that, apart from data suggesting serious harm/toxicity (which none of the RCTs have shown).
I'm very curious as to people's thoughts about all this and look forward to hearing from you in the comments!
Drew Rosielle, MD is a palliative care physician at the University of Minnesota Health in Minnesota. He founded Pallimed in 2005. You can occasionally find him on Twitter at @drosielle. For more Pallimed posts by Drew click here.